Microbiological analysis
- R&D&I Programmes
- Technological Services
- Consultancy Services
- Laboratory Services
- Training and Events
- Sectors
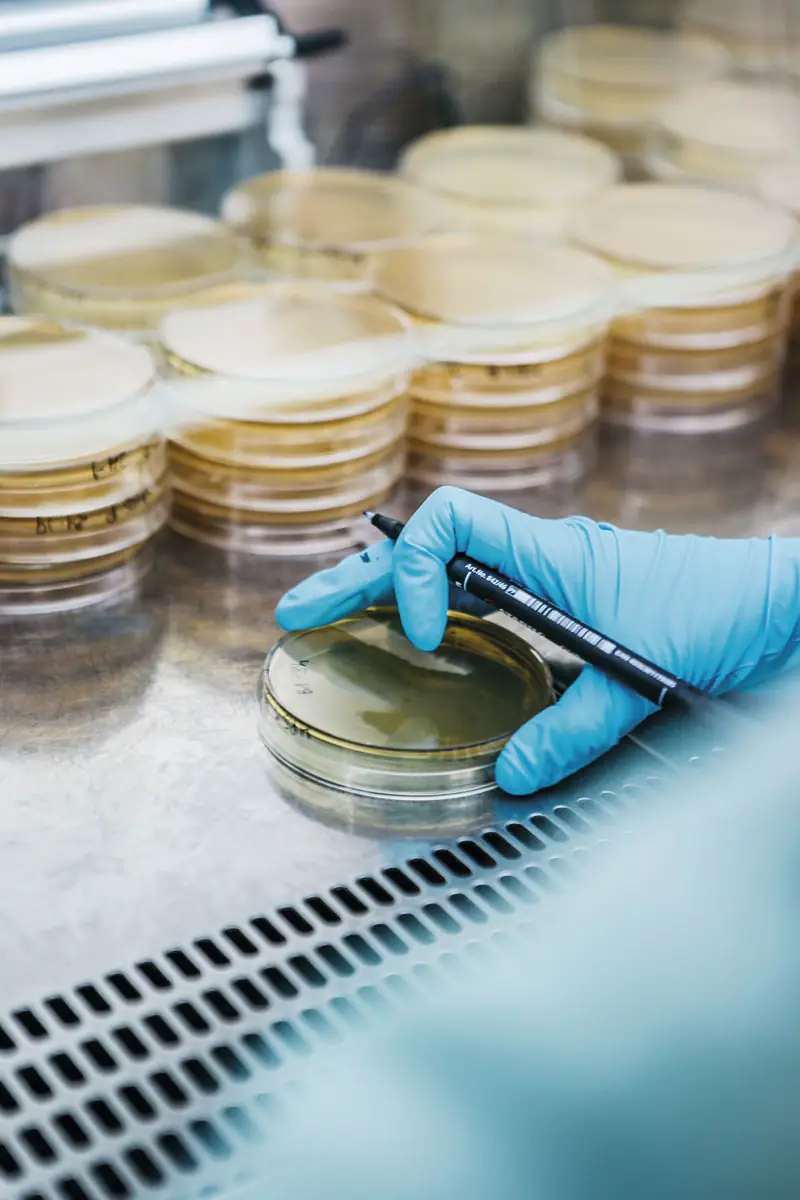
Microbiological contamination, especially bacterial contamination, is the most common cause of foodborne health problems. Under ideal conditions, all foods should be free from the presence of pathogenic microorganisms. But achieving this is not always possible. Preventive measures must be taken to avoid product contamination and pathogenic microorganism growth in products. However, analytical control is also an irreplaceable tool to ensure food safety.
Control plan design must be based on a detailed hazard analysis. From this, we can determine the required analyses and their frequency in order to control necessary critical points. In order to ensure batch-to-batch safety, some companies implement positive release systems, which consist of blocking the release of production batches until each batch has received analytical confirmation of its safety.
In order to ensure a high level of public health protection, Regulation (EU) No. 2073/2005 establishes the microbiological criteria applicable to foods. The food companies must comply with these criteria throughout the entire food chain, from the manufacturing process to the shipping of the product. This regulation states that food products must not contain microorganisms, toxins or metabolites in quantities that pose an unacceptable risk to human health.
In order to meet these needs, food operators must use analytical services of the highest quality. Accreditation is a decisive factor in this respect, and the control services must also be quick and effective. This is especially important when implementing control systems with positive batch release.
| Responsible | AINIA |
| Address | Calle Benjamín Franklin, 5 a 11, CP 46980 Paterna (Valencia) |
| Purpose | To attend to, register and contact you to resolve the request you make to us through this contact form |
| Legitimation | Your data will be processed only with your consent, by checking the box shown on this form |
| Recipients | Your data will not be transferred to third parties. |
| Rights | You have the right to request access to, correct or delete your data. You can also request that we limit its processing, oppose it and request the portability of your data by contacting our postal address or [email protected] |
| More info | You can find more information in our Privacy Policy |
| DPO | If you have any questions about how we will treat your data or would like to make any suggestions or complaints, please contact the Data Protection Officer at [email protected] or at the Data subject support form |
I consent to the use of my personal data to process my request, as set forth in your Privacy Policy.
I consent to the use of my data to receive information and commercial communications from your entity.
