Health
- R&D&I Programmes
- Technological Services
- Consultancy Services
- Laboratory Services
- Training and Events
- Sectors
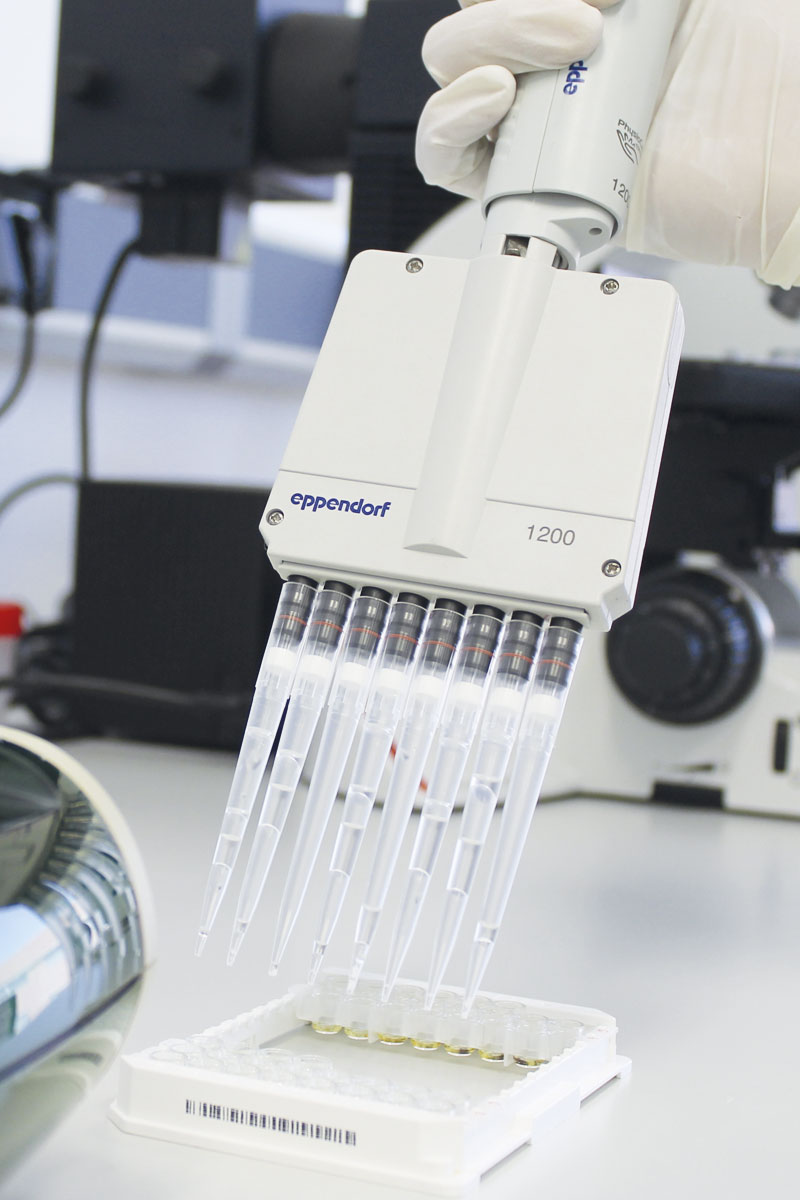
Constant search for ingredients and active ingredients which make the company more competitive in the market. Interest in sustainably sourced alternative compounds that respect safety requirements.
To verify compound performance using techniques alternative to animal studies, guaranteeing that the expected effect is developed.
Development of active pharmaceutical ingredients using supercritical fluids. In one single extraction and purification step, we extract high concentrations of the active ingredient with high purity. These processes are clean. They leave no waste or pollutants, so there is no need to manage waste or by-products.
On the other hand, we design biosynthesis processes starting with the selection of microorganisms for the industrial production of active ingredients with functional activity. Biofactories are also used to obtain molecules such as active pharmaceutical ingredients. We have our own collection of more than 400 natural strains with various functionalities.
We design microencapsulation processes to guarantee the protection and controlled release of the active ingredients. This ensures that they develop the intended biological activity.
We have a comprehensive in vitro model that is able to study the release and absorption of active ingredients for formulation into pharmaceuticals. Thus, we are able to obtain more biorelevant preclinical data than with standardised analytical methods.
We have a platform to assess intestinal permeability and absorption of bioactive ingredients (APIs). We use an intestinal epithelial cell model to reproduce intestinal absorption dynamically. We employ organ-on-chip technology, based on the use of microfluidic platforms to faithfully reproduce an intestine on a chip.
We complement this dynamic cellular platform with our in vitro models to simulate gastrointestinal digestion for pharmacopoeia. This enables us to improve and optimise the formulation and development of new galenic formulations. We faithfully simulate oral, gastric and intestinal digestion (small intestine and intestinal microbiota of the colon) to study the resistance and absorption of bioactive compounds, as well as for drug dissolution studies.
| Responsible | AINIA |
| Address | Calle Benjamín Franklin, 5 a 11, CP 46980 Paterna (Valencia) |
| Purpose | To attend to, register and contact you to resolve the request you make to us through this contact form |
| Legitimation | Your data will be processed only with your consent, by checking the box shown on this form |
| Recipients | Your data will not be transferred to third parties. |
| Rights | You have the right to request access to, correct or delete your data. You can also request that we limit its processing, oppose it and request the portability of your data by contacting our postal address or [email protected] |
| More info | You can find more information in our Privacy Policy |
| DPO | If you have any questions about how we will treat your data or would like to make any suggestions or complaints, please contact the Data Protection Officer at [email protected] or at the Data subject support form |
I consent to the use of my personal data to process my request, as set forth in your Privacy Policy.
I consent to the use of my data to receive information and commercial communications from your entity.
